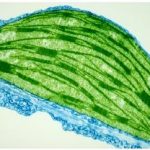
Pigments and Photosynthesis – Make Up Module
Read along in your lab handout/lab manual with the information in this webpage. Fill in and answer all questions in Activity 1, Activity 2 and at the end of the lab unit accordingly. Your lab handout will be collected and graded later.
Photosynthesis Lab Handout(opens in new window) – Includes areas for filling out your tables and answering questions.
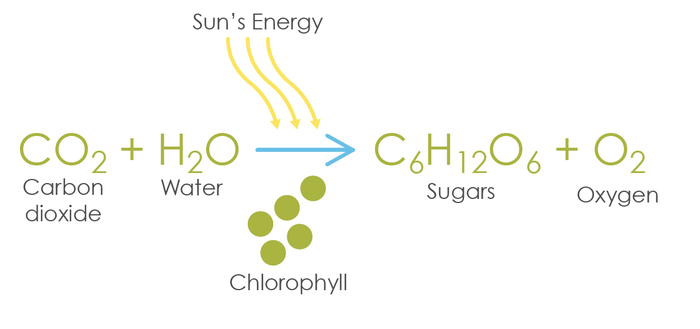
(Balanced equation 6CO2 + 6H2O –> C6H12O2 + 6O2)
Leaf Anatomy and Photosynthesis:
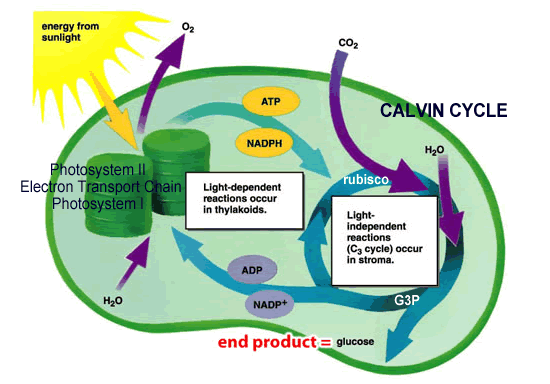
Leaf Cross section
Structure and Working of Stomata (3:50) – animation
Stomata from BIO 183 student prepped Zebrina epidermal whole mount and leaf peels (A, B, and C)
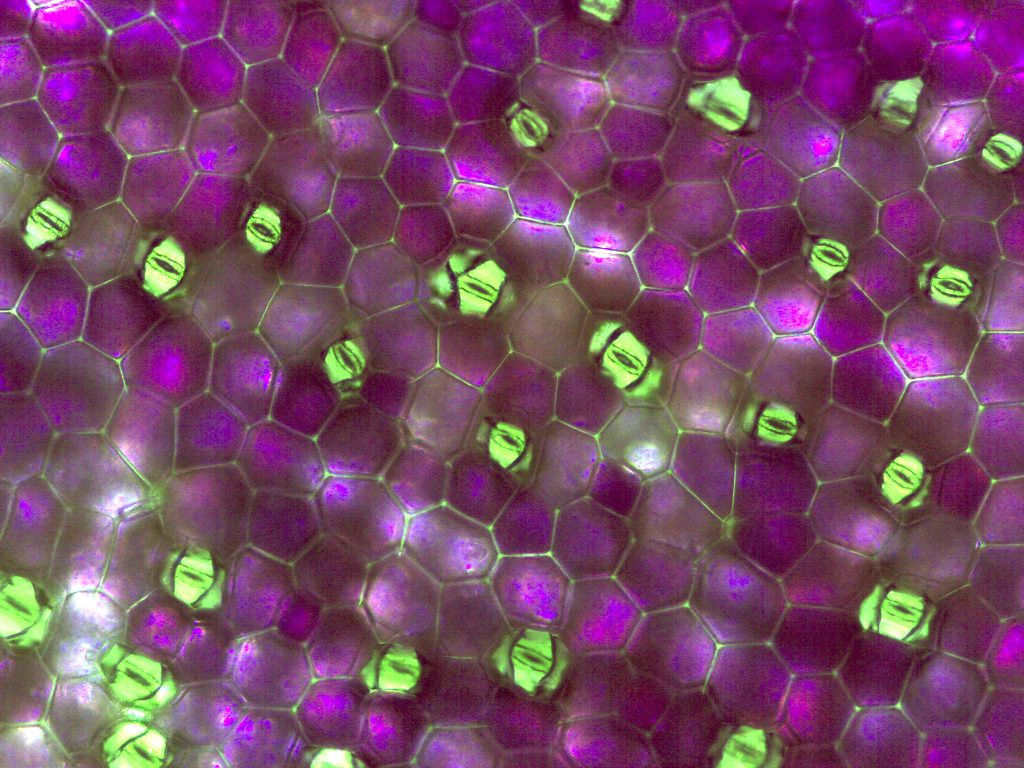
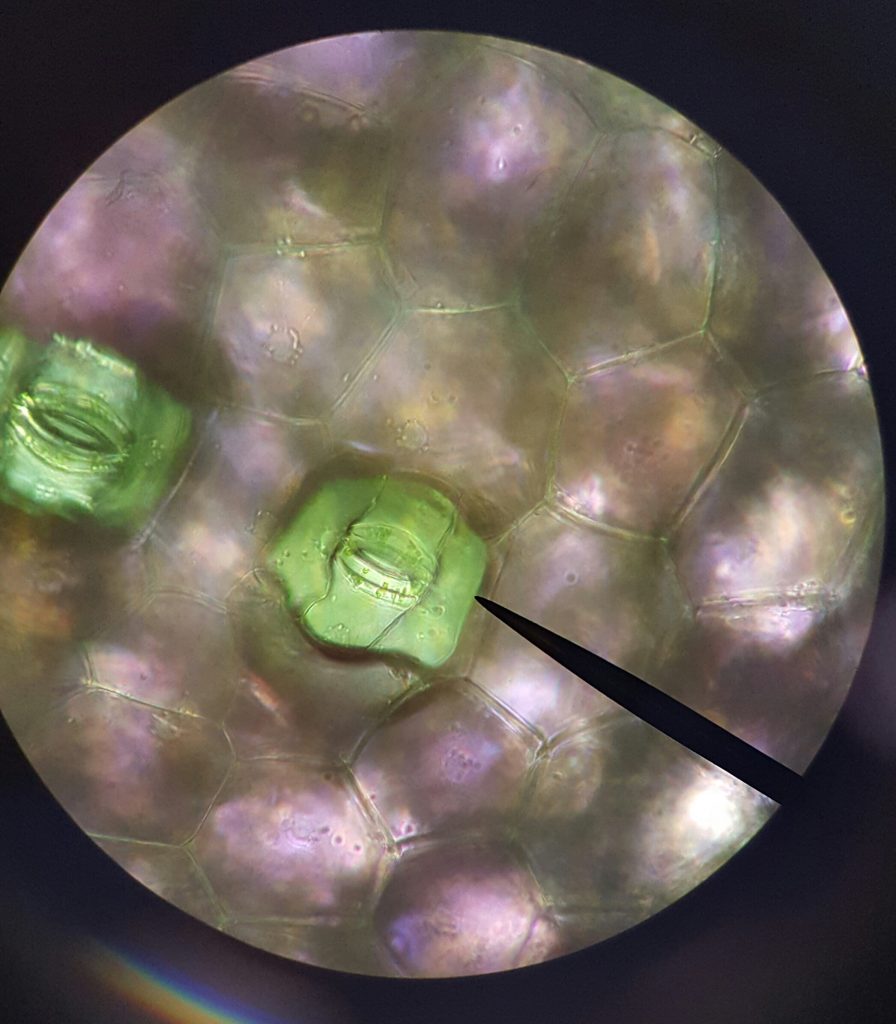
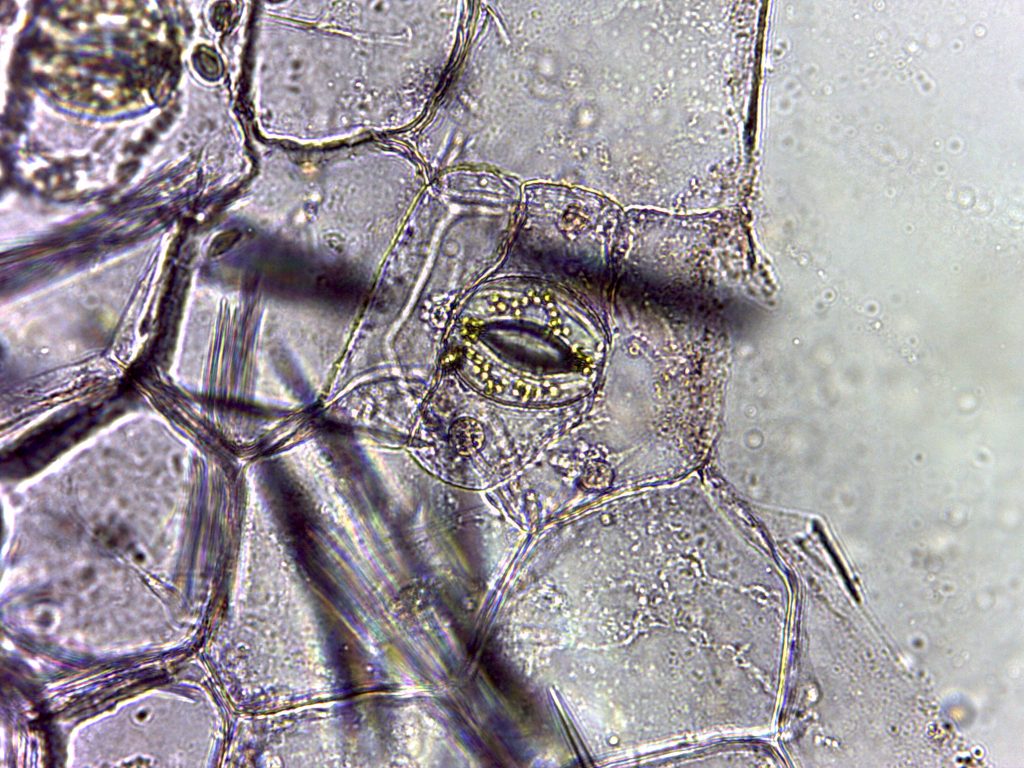
A. Zebrina lower epidermis, 40X magnification. B. Zebrina lower epidermis, 100X magnification, note guard cell shape creating the stoma/opening. C. Zebrina lower epidermis, 400X magnification, note chloroplasts in the guard cells.
Computer animation as you travel into a leaf, plant cells, and into a chloroplast.
What types of cells can you see inside the leaf? Once in a cell, what organelles do you see? Once in the chloroplast, what structures can you see? What rotating molecules are embedded in the surface of the grana? Where else have we seen this special molecule?
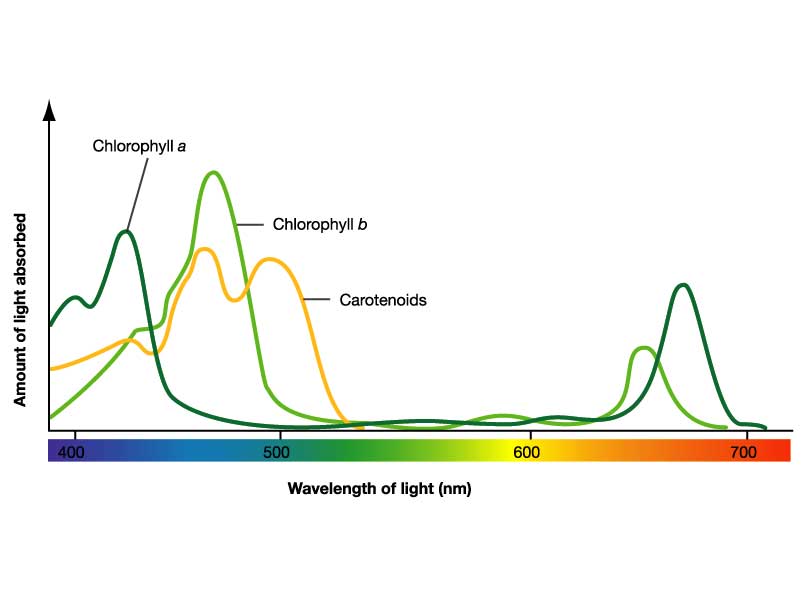
Visible Wavelengths of Light and Absorbance Spectra of PS Pigments
Activity 1: Paper Chromatography
Plant Pigment Chromatography – review your lab handout/lab manual for the key concepts of the procedure that produced the following images.
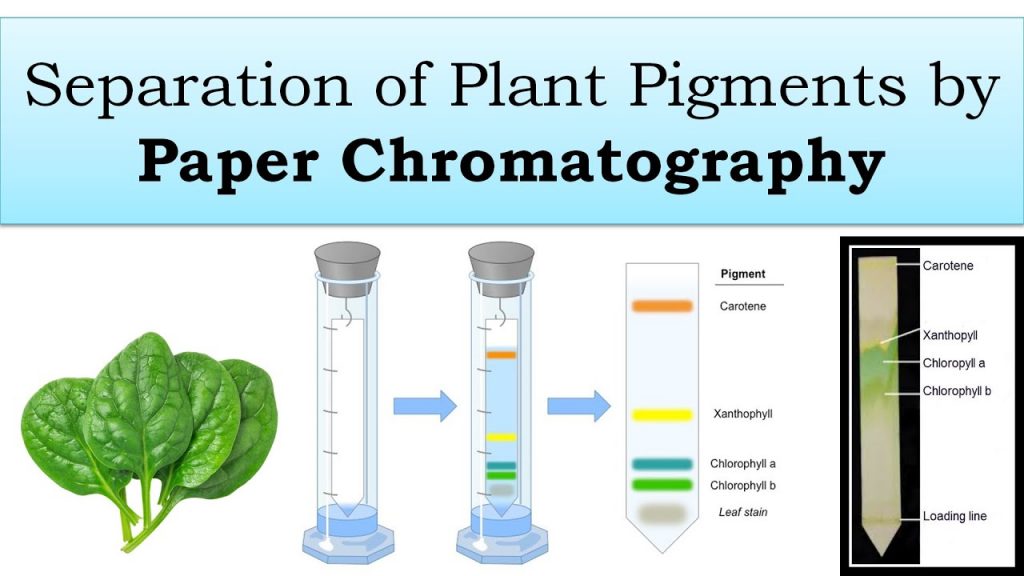
Photosynthetic Pigments in Spinach – separation by a similar technique that we used with magnolia leaves.


A. A coin is used to crush the Magnolia leaf and embed the chromatography paper with leaf tissue.
B. The chromatogram placed into a chamber with a 9:1 Ether Acetone solvent. Within 5-10 minutes you should start to see 4 distinct photosynthetic pigments separating as the solvent is absorbed up the paper.
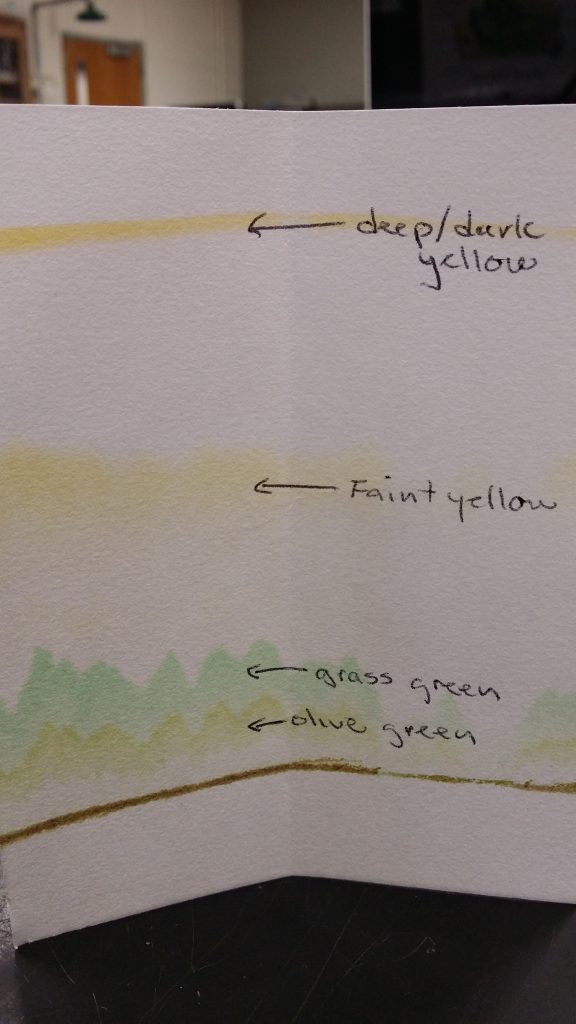
C. The same chromatogram after 30 minutes and dried. You can see 4 distinct photosynthetic pigments. Can you tell which pigment is the primary pigment, chlorophyll a? Which are the accessory pigments chlorophyll b, xanthophyll and beta-carotene? What is the bottom brownish line composed of? Sketch and name all of these colored compounds in your lab handout.

Above (D) is a new chromatogram that only ran for 15 minutes. You can see the pigments have not separated as much form each other as in the 30 minute chromatograms above in C. D is a picture taken with regular room lights (white light).
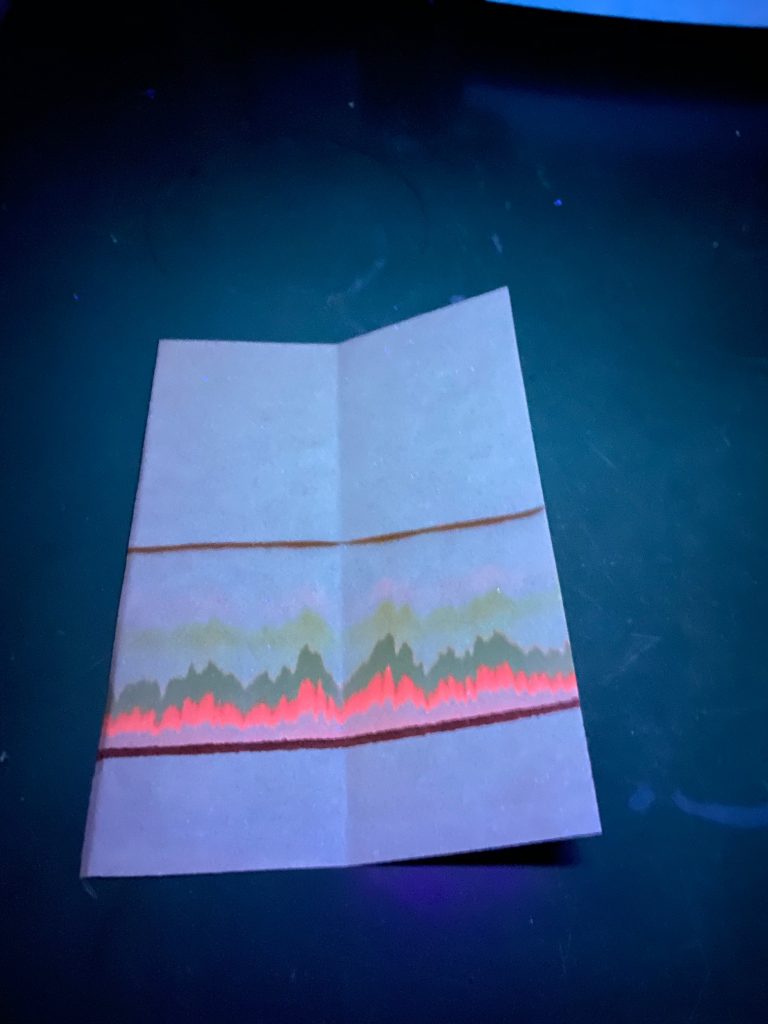
E. Is the same chromatogram as seen under UV light. The lower green pigment above the origin line seems to be glowing a red color (it is fluorescing!). What does this infer about the green pigment, light energy, and the pathway of the photosynthetic reactions?
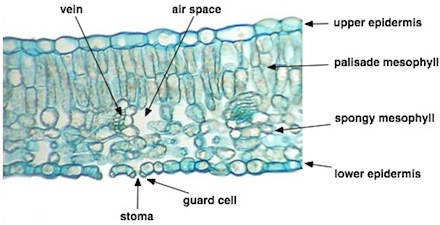
Activity 2: Light Reaction Measurements – Background information:
Light Reactions of Photosynthesis – a nice animation explaining how ATP and NADH are produced.
A new Animation of Light Reactions –more in depth than above or needed for lab, but may be helpful for lecture.
Light Reactions Diagram below
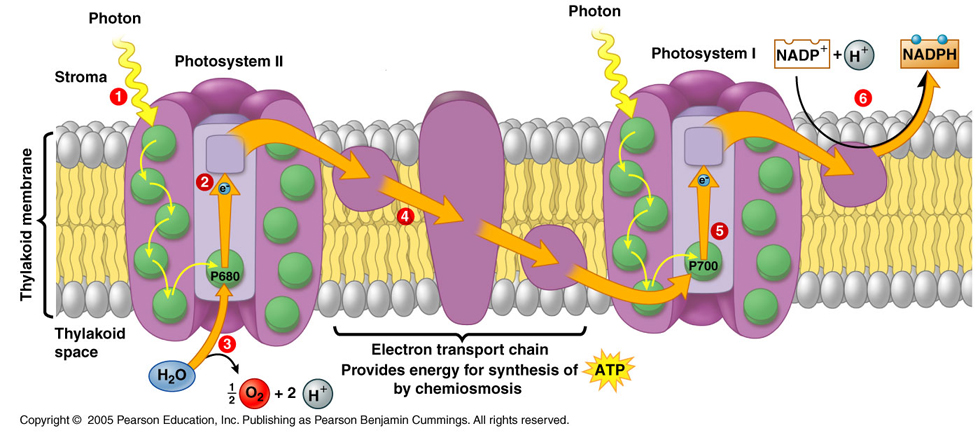
We will study the light reactions of photosynthesis by using an artificial electron acceptor (DPIP) with isolated spinach chloroplasts and a source of light. As electrons move along in the Light Reactions of Photosynthesis, DPIP can be used to capture electrons. DPIP is dark blue, but will become more clear as it accept/intercepts electrons moving along in the Light Reactions. By measuring the change in color (from blue to more clear) we can quantify the rate of the Light Reactions of photosynthesis. We will use a spectrophotometer to monitor this reaction by monitoring the Transmittance of light passing through the DPIP/chloroplast solution. This experiment is referred to as the Hill Reaction.
View this video for the Hill Reaction procedure being done for an AP Biology class. The whole video is 12:54, time 0-7:10 minutes provides good background for the experiment and set up.
- The samples are prepared the same way we would have done in lab. You may want to take notes.
- Pay close attention to the Blank (#1) and the White Light Treatment (#3). The other 2 samples in the video (Dark (#2) and No Chloroplasts (#4) are not a part our our experimental choices as they show predictable results).
- In our experiment we collected data every 30 seconds for 6.5 minutes, not every 5 minutes over 20 minutes.
- The video also shows the use of an older model Spec 20 and not the newer more automatic Genesys 10S spectrophotometer models that we used in our Enzyme lab and Photosynthesis lab. Both types of spectrophotometers can measure: A (Absorbance) and T (Transmission) and work in similar ways.

Note the difference in blue color in these test tubes. The tube closest to the brown DPIP bottle is the blank (no DPIP added). The more that light reactions of photosynthesis occur, the lighter or more clear the test tubes with DPIP and thylakoids will become. The darker tubes show less reaction.
Follow the directions in Activity 2 in your lab handout and answer the questions in the lab handout.
Reminders about the Spectrophotometers we used in the Enzyme lab:
How Spectrophotometers Work – this week we will measure Transmittance, not Absorbance as measured in the Enzyme lab.
How to use a Genesys 10 Spectrophotometer (4:43) -same video as on the Enzyme Lab webpage.
REMEMBER – your BLANK is very important. It contains all the components of the experiment except for the color indicator that will change color as the reaction proceeds. The BLANK is used to set up your Spec to monitor the changes in color that occur in your sample tube. By pressing “Measure Blank” with your Blank in the appropriate space, the machine will zero itself to not “measure” those components. When you change to a treatment cuvette the Blank component measurement is automatically subtracted out and only the change from the reaction seen in DPIP will be measured. Watch the above video if you are still unclear about how to use a Spec and purpose of using a Blank.
List of Possible Light Treatments for our Photosynthetic Experiments
Below are some provided pictures of the set-up we would have in our labs and some of the treatments available. Data sets are be provided below the images. You will select or be assigned ONE of the data sets that explore 2 additional treatments beyond white light to add to your lab manual. You will need to graph ALL of the data (the provided white light data and 2 treatments) in one graph. Be sure to include the slope equations and trendlines for each of the lines on your graph.

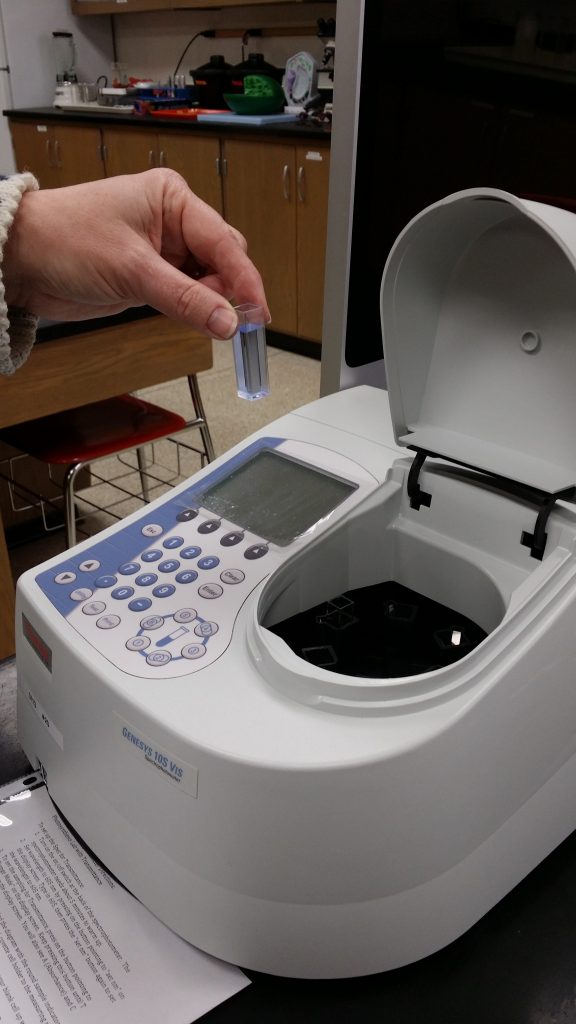
A. The above pictures show the set up of a cuvette at a set distance from a lamp shining white light. The fishbowl acts as a heat sink between the lamp and the cuvette. B. The cuvette going into the Genesys spectrophotometer to measure Transmittance every 30 seconds.
The last 4 pictures below, (C-E) show the use of colored films to alter the wavelengths of light reaching the sample and (F) an assortment of different light bulbs to use (see the list below for bulb specifics)




Provided Data: Choose one data set to use for the Photosynthesis Graphing Activity.
Photosynthesis Graphing Activity: Construct ONE graph containing all of your data:
- You should also include a copy of all your data and your graph in your lab handout.
- Include a trendline for each sample and add the slope equation for each of the trendlines on your ONE graph.
- Be sure to include all 5 key components of a proper graph (see the Resources page Appendix D. if you are unsure of this).
The Calvin Cycle (light independent reactions) – a nice animation overviewing these reactions, though not a main focus of our lab.
Cool Article on Biohybrid Light Antennas – Altering natural light harvesting antennae (pigments) to maximize the range of useable light in photosynthesis. Working towards increasing solar energy capture systems.
The Biochemistry of Fall Foliage – plants saving/reclaiming resources
Fall Foliage Outlook 2022: When will leaves change in NC? -NCSU College of Natural Resources
A New Way to Genetically Tweak Photosynthesis Boosts Plant Growth – Further explanation of the metabolic pathways involved can be found in the primary reference article of this research.